Blood is amazing. It carries oxygen and nutrients from the core to the extremities. It fights off pathogens and other invaders to keep our bodies healthy. And it rushes to the site of an injury to stop damage through a process called the coagulation cascade.
Blood touches every organ and every aspect of human health and disease, but sometimes blood itself can be the root of disease.
Coagulation (clotting) is a complex mechanism. Too much clotting is a problem, leading to blocked vessels, strokes, heart attacks, or other fatal complications.
Too little clotting is also a problem. Bleeding disorders, such as hemophilia or von Willebrand disease, impact the blood’s ability to clot, leading to excessive (and sometimes spontaneous) bleeding.
March is Bleeding Disorders Awareness Month, and Bloodworks is proud to support patients with these conditions through our lifesaving research.

Hemophilia is one of the best known bleeding disorders, perhaps due to its association with European royalty or Ryan White’s tragic fight with AIDS. It’s a genetic condition caused by deficiencies in coagulation factor within the coagulation cascade and causes excessive bleeding, particularly in joints and muscles, and with trauma or surgery.
Contrary to popular belief, people who live with hemophilia tend to have the same response to minor skin injuries (like paper cuts) as the rest of the population because the body can stem those small wounds without the clotting mechanisms missing in hemophilia.
There are two main forms of hemophilia: hemophilia A (HA) and hemophilia B (HB), each caused by deficiencies in coagulation factor VIII (FVIII—pronounced “factor eight”) or coagulation factor IX (FIX—pronounced “factor nine”), respectively.
The DNA changes that cause hemophilia occur in the F8 gene in HA or in the F9 gene in HB on the X-chromosome. Because of this chromosomal location, symptoms tend to be more severe in people with only one X chromosome, which is most commonly an XY chromosome pair (“male”), but (despite what medical science thought for decades) people with two X chromosomes (usually “female”) often present symptoms as well.
Hemophilia may be mild, moderate, or severe, depending on how much the level of factor is impacted.

Today, most hemophilia patients can live normal lives with modern treatment, typically at-home infusions of the specific clotting factor they are deficient in.
Because hemophilia is a genetic disorder, genetic testing (genotyping) provides useful information for clinical care. However, in 2012, only 20% of U.S. hemophilia patients had their gene sequenced.
This significant, unmet need led to the formation of a national hemophilia genotyping program called My Life, Our Future (MLOF), led by Bloodworks’ Dr. Barbara Konkle as primary investigator. The program offered free genetic testing to hemophilia patients and built a biorepository as a research resource for the future.
Bloodworks’ Jill Johnsen, MD now stewards the MLOF biorepository.
MLOF was a collaborative effort between Hemophilia Treatment Centers (HTCs) — including the Washington Center for Bleeding Disorders, formerly part of Bloodworks — the American Thrombosis and Hemostasis Network, the National Hemophilia Foundation, and Bloodworks Research Institute.
From 2013 to 2017, more than 100 treatment centers around the United States enrolled 11,341 people (69% male and 31% female) either with hemophilia or at risk for having inherited a gene for hemophilia for testing for HA, HB, and unspecified variants. Over 9,000 of these subjects elected to participate in research.
Bloodworks collaborated with the University of Washington to create a new genotyping method for MLOF. Bloodworks’ Research Institute processed every submission and Bloodworks’ clinical Genomic Testing Laboratory validated and returned results to providers.
Bloodworks recently completed the analysis of the MLOF program, and initial results both answer and pose intriguing questions.
Some hemophilia patients develop immune responses called inhibitors to factor drugs that limit their treatment options and make bleeding harder to manage.
“Sometimes, probably because they’re not making the same version of the factor we’re giving them, their immune system sees [factor treatment] as foreign and they’ll make a response to it,” explained Dr. Johnsen, “And we call [that response] an “inhibitor” because it’s inhibiting the factor we gave them. It’s making it so that [treatment]’s not effective.”
We don’t fully understand why some patients develop inhibitors and some do not, largely because hemophilia is rare and we haven’t had the data. However, given that inhibitors tend to run in families, there is likely a genetic component to inhibitor development.
“One of the risks for inhibitor is the gene change that led to hemophilia in the first place, and it’s probably somewhat based on are you making any normal factor at all? Is [the treatment] a completely foreign protein?” Dr. Johnsen said. “And we aren’t going to be able to understand that better until we classify what are the genetic changes associated with high inhibitors versus not.”
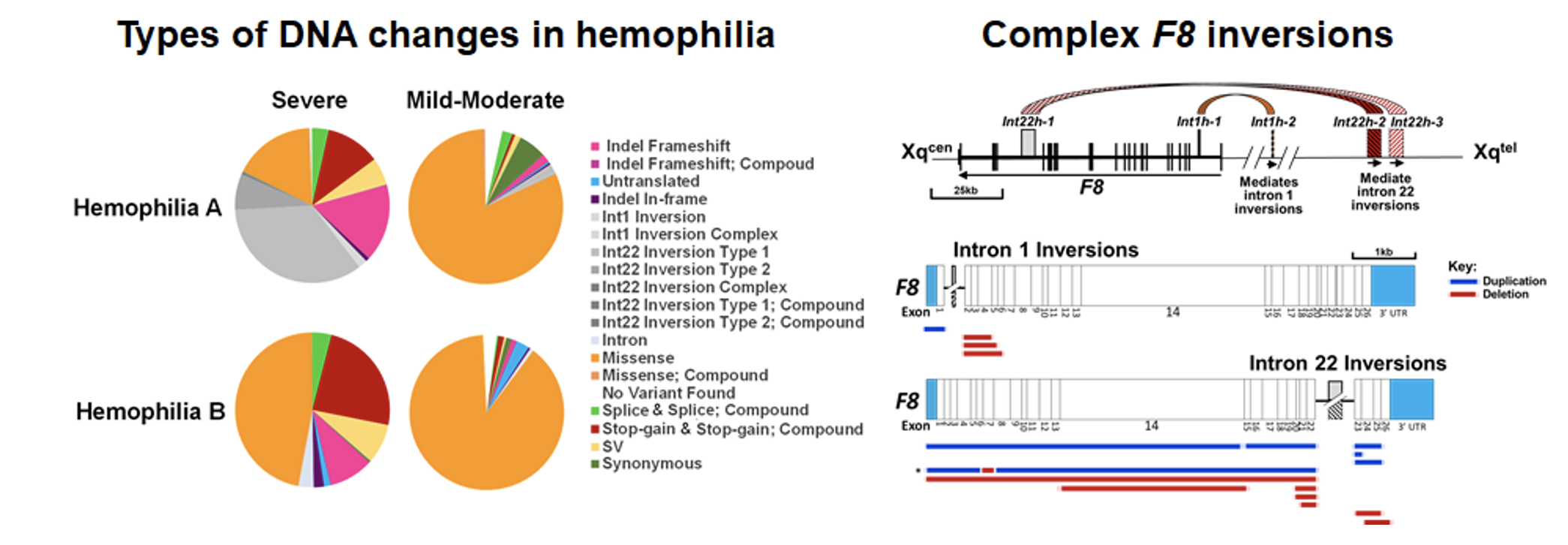
DNA variants were detected in roughly 98% of male MLOF participants with HA and HB. The study found a total of 1914 unique DNA variants, of which ~38% were previously unknown.
“What we’ve spent some time doing is classifying the [hemophilia-causing] genetic change vs whether or not they had ever had an inhibitor in that clinical data set,” Dr. Johnsen explained. “And hopefully that will consolidate a nice foundation for immunologists to then go work in this data set and understand what are the other genes that participate in inhibitor development.”
Our genes are significantly tied to our biological ancestry, as approximated by the census categories of race and ethnicity. Race and ethnicity are social constructs that lead to social determinants of health but do often reflect genetic changes.
MLOF data showed that race and ethnicity are associated with inhibitor occurrence. Inhibitors were higher in HA and HB in subjects who self-identified as Black (compared to white) and in subjects who self-identified as Hispanic or Latinx compared to those who did not.
There were also high inhibitor rates in subjects self-identifying as Native American and Pacific Islander, but numbers were too small to draw further conclusions.
Dr. Johnsen acknowledges that the possible reasons there are racial and ethnic differences in inhibitor formation is complicated, “there’s a genetic component, but also undoubtedly many other components.”
For example, Dr. Johnsen shared that we know that there are inequities to access to healthcare in the United States. Everyone involved in the study received care from an HTC, so they should be getting similar levels of care in regards to hemophilia, though may not be getting the same treatment in other aspects of health.
“There are a lot of socio-economic and environmental things that really can’t be separated because we have so much inequity in our society,” she explained. “we know that there are other things that predispose to inhibitor development – if you’re baseline inflamed or you have other exposures, it’s plausible that that is a reason.”
“We don’t want to say it’s someone’s genetic make-up that’s causing this risk, but more importantly there’s a whole group of people that self-identify in any number of categories that have higher risk, and that’s not okay, and we need to work on it.”

Approved researchers within the scientific community are currently using the biorepository to investigate areas like factor half-life, inhibitors, and additional clotting factors.
“Hopefully [MLOF] will become the basis to support even more ongoing research,” Dr. Johnsen said. “we’ve got tons of questions can be asked with this repository, we’re developing new data in this repository all the time.”
She added, “We’re hoping this repository can even be linked to other research studies.”
Dr. Johnsen herself is one of these researchers: her lab is now using the MLOF research repository and an award from the Hemostasis and Thrombosis Research Society to investigate how genes and factor level impacts bleeding in females.
“We have lots of reason from prior work to know that in women, the [factor] level doesn’t correlate with bleeding like it does in men, and we don’t have a great explanation, but what does correlate is that they have the genetic change that causes hemophilia,” she said.
“Our approach is, let’s go back to basics: what’s the one thing they have in common if they’re bleeding excessively if they have this gene change? And then to say can we better understand what’s being expressed from the abnormal gene versus the normal gene? Can we start to figure out why the factor levels aren’t correlating? And just have a better understanding of how much bleeding these women having.”
Your financial contributions support Bloodworks’ lifesaving blood research.

Comments
Share your thoughts →How genotype testing could benefit people with hemophilia
[…] is investigating how genes and factor levels affect bleeding in women. In 2022, she told Bloodworks, a medical laboratory company, that, “we have lots of reason from prior work to know that in […]
Tell Us What You Think!